ACIDIFICATION
- Ocean surface pH declined from 8.2 to below 8.1 over the industrial era as a result of an increase in atmospheric CO2 concentrations. This decline corresponds to an increase in oceanic acidity of about 30%. Reductions in surface water pH are observed across the global ocean. Ocean acidification has impacts on marine organisms and has already affected the deep ocean, particularly at high latitudes. Models project further ocean acidification worldwide. The target under
United Nations
Sustainable Development Goal
14.3 is to minimise the impacts of this by 2030.
The Mediterranean basin receives a considerable amount of anthropogenic carbon from the North
Atlantic Ocean through the Straits of Gibraltar
(SOG), as a continuous cycle.
• The whole water column of the Mediterranean Sea is invaded by
anthropogenic carbon (CANT) with concentrations ranging between 35.2 and 101.9 µmol kg.
• Strong CANT invasion (>60 µmol kg) observed at the intermediate and deep layers in the Alboran, Liguro- and Algero-Provencal Sub-basins in the Western basin, and in the
Adriatic Sub-basin in the Eastern basin. • Mediterranean Sea waters are already acidified, especially those of the Western basin. • The acidification varies between −0.055 and −0.156 pH unit in the Mediterranean Sea. • Despite the high acidification levels, Mediterranean basins are still highly supersaturated in both calcite and aragonite. Carbon
dioxide (CO2) emissions from fossil fuels burning and land use change since the industrial revolution have caused a considerable increase in atmospheric CO2
concentrations. Recent investigations have estimated that cumulative emissions of CO2 have reached in the period from 1870 to 2013 about 535 ± 55
GtC. However, a significant CO2 amount has been captured from the atmosphere by natural sinks, such as the terrestrial biosphere and the ocean. In particular, the global oceans have absorbed about 30% of the anthropogenic carbon emissions over the past 200
years.
The withdrawal of CO2 by the oceans has, however, drastic consequences for the marine environment, as it originates a rise in average surface ocean concentration of H+ that leads to a pH decrease in seawater and a range of chemical changes known collectively as “the other
CO2 problem” or the ocean acidification (OA)
phenomenon. The impact of OA on marine biogeochemical cycles and biota has been well documented by laboratory studies and already observed to occur in certain ocean
areas. It has been suggested that the Mediterranean Sea (MS) represents one of the world’s most sensitive ocean regions to increasing atmospheric CO2 and the subsequent OA. Nevertheless, contradicting observations of pH changes with time have been reported in the basin. A recent study affirms that the MS is already acidified, although distinct OA rates are provided depending on the degree of
anthropogenic carbon accumulation by a particular sub-basin, with regional pH decreases oscillating between −0.055 to −0.156 pH units with respect to the
pre-industrial levels and the anthropocene
age. On the other hand, another work points out to pH reductions between 0.005 to 0.06 pH units due to the anthropogenic carbon storage in the basin during the same
period. Moreover, it has been proposed that the pH decline would be amplified in the MS due to its higher capacity for CO2 absorption in relation to open ocean regions and the relatively short ventilation times of its
water
masses, statements that were challenged recently by a modelling
approach which indicates that the average anthropogenic change in surface pH does not differ significantly from the global-ocean average.
Considerable efforts have been made over the last decade to characterize the carbonate system in Mediterranean water
masses, to explore how much anthropogenic CO2 has been taken up by this semi-enclosed sea and to assess the corresponding pH diminution. Most of these studies rely on discrete data sets that extend over a specific period of time or cover a particular Mediterranean sub-region. Thus, sometimes, these studies lead to discrepant conclusions when comparisons between data acquired in different periods, using distinct techniques and/or in distant locations are made.
The assessment of the marine ecosystems responses to the oceanic CO2 uptake requires then sustained observations that provide the needed high frequency data about changes in
ocean chemistry. At present, this type of measurements is being collected in a few ocean time-series. The continuous monitoring of the carbon system parameters (and other tracers for hydrography and biochemistry) in these key sites have supplied relevant information on the ocean CO2 sink and the derived pH changes in different regions, confirming a general OA trend over the past two decades.
Sustained time-series observations also started in the SOG a decade ago through the establishment of the GIFT (Gibraltar Fixed Time Series) observatory, since this region represents a privileged site to observe the evolution of the Mediterranean waters over time. This narrow channel (14 km wide in its narrowest section) is the only connection of the MS with the North Atlantic, thereby playing a major role in the global circulation and biogeochemistry of the basin.
The circulation pattern in the SOG has been traditionally described as a two-layer system, with surface Atlantic water (AW) flowing eastwards to the MS and the Mediterranean Outflow Water (MOW) moving westward to the Atlantic Ocean underneath. The AW enters the MS and flows clockwise in the Alboran Sea. Subsequent surface circulation patterns at the basin level are influenced by deep and intermediate water formation driven by strong winds, which is in turn affected and amplified by topography. Deep and intermediate waters are formed in four major areas: the Levantine Basin, the source of the Levantine Intermediate Water (LIW); the Gulf of Lions where the Western Mediterranean Deep Water (WMDW) is formed; and two adjacent regions, the Adriatic and the
Aegean
Seas, which together merge to form the Eastern Mediterranean Deep Water (EMDW). The MOW that leaves the basin through the Strait of Gibraltar is then a mixture of these intermediate and deep waters, fundamentally LIW, which flows across the Strait of Sicily into the Western Mediterranean basin and the WMDW, which occupies the bottom layer. The contribution of the AW that penetrates into the MS in surface to the final outflow exiting the basin is negligible. By monitoring the hydrography and biogeochemistry of the MOW in the SG, the history and evolution of the main intermediate and deep Mediterranean
water masses can be examined.
The exchange of waters of different ages carrying diverse concentrations of biogeochemical properties in the S0G also influences global inventories in the two neighbour regions. Regarding the marine carbon cycle, a net transport of anthropogenic carbon from the Atlantic towards the Mediterranean has been identified, which has been indeed responsible for 25% of the basin-wide CO2 uptake over the last 200
years.
In this work, the authors use pH measurements taken at the GIFT at a high sampling rate to assess temporal trends of pH change in Mediterranean waters. Data were obtained by autonomous sensors installed in a mooring line deployed at the Espartel Sill, which has been proven to be the most suitable section in the
SOG for monitoring the MOW. Results presented here correspond to the first continuous pH records at a high temporal resolution registered in the channel from August 2012 to June 2015.
This work provides the first rates of pH decrease in the MOW and in its forming water masses separately, which can be considered indicators of OA in the basin, confirming previous evidence. In addition, by using a simple model,
they present a tool for tracking pH and its temporal variability in the MS. The
authors: Susana Flecha, Fiz F. Pérez, Jesús García-Lafuente, Simone Sammartino, Aida. F. Ríos & I. Emma Huertas 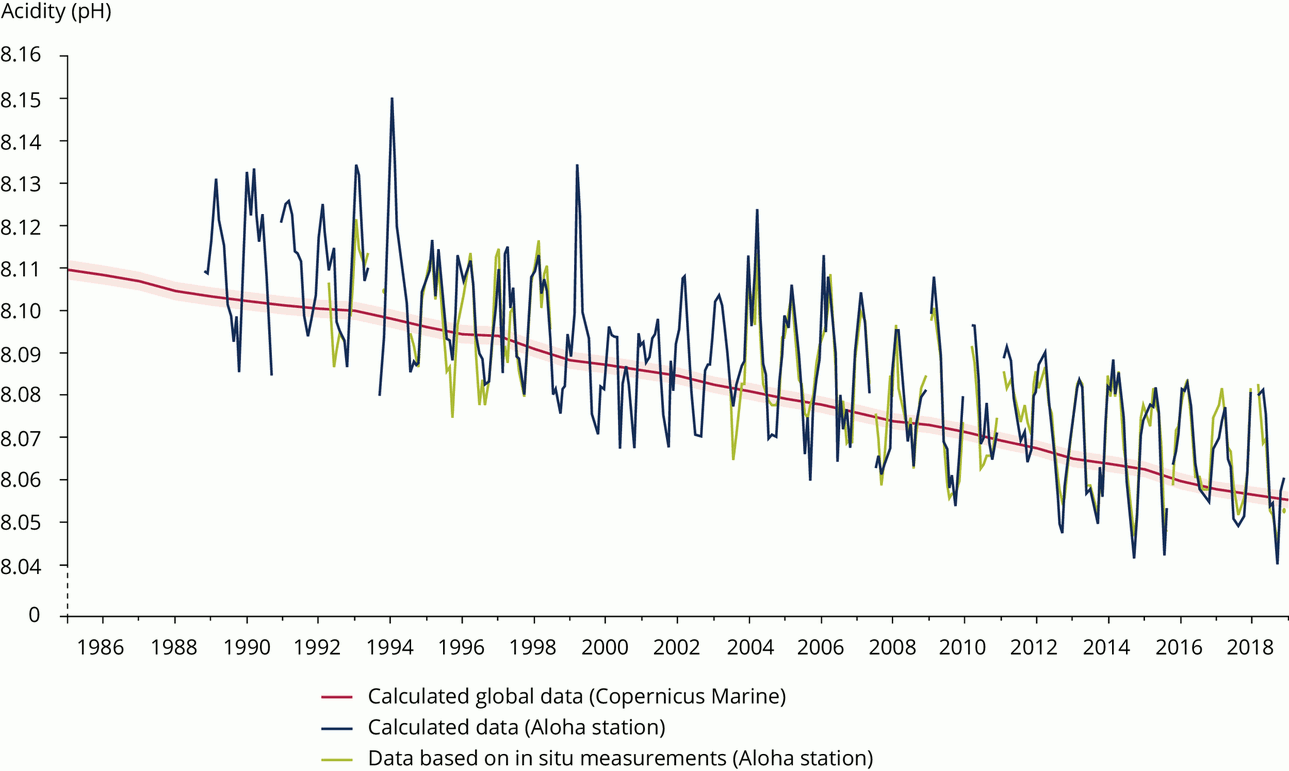
ABOUT
OCEAN ACIDIFICATION Over the last million years, average surface seawater pH has been relatively stable, oscillating between 8.3, during cold periods (e.g. during the last glacial maximum 20,000 years ago), and 8.2, during warm periods (e.g. just prior to the industrial revolution). Rapid increases in atmospheric CO2 levels due to emissions from human activities are now threatening this stability, as emitted CO2 is partially absorbed by the ocean, causing a decline in pH and ocean acidification. A goal of the 2030 Agenda for Sustainable Development is to address the impacts of this.
The global annual mean atmospheric CO2 concentration exceeded 400 ppm in 2016, which is more than 40% above the pre-industrial level (280 ppm); half of that increase has occurred since the 1980s. Over the same period, ocean pH decreased from 8.11 to below 8.06, corresponding to an approximately 30% increase in acidity. This decrease in pH occurred at a rate about 100 times faster than any change in acidity experienced during the past 55 million years.
This indicator looks at the longest time series of measured pH values available, from the Aloha station, offshore of Hawaii, and also calculated data on global average surface ocean pH from the Copernicus Marine Environment Monitoring Service (CMEMS). The reduction in pH measured in surface mixed-layer depths (up to 100 m) is consistent with that calculated from atmospheric CO2 concentrations, assuming thermodynamic equilibrium between the ocean surface and atmosphere. The northernmost seas, i.e. the Norwegian and Greenland Seas, have seen significantly larger decreases in pH than the global average.
Average surface open ocean pH is projected to decline further, with the largest projected decline representing more than a doubling in acidity. This will affect many marine organisms and could alter marine ecosystems and fisheries. Such rapid chemical changes are an added pressure on marine calcifiers and Europe’s sea ecosystems.
Without substantial reductions in CO2 emissions, it will take thousands of years for the Earth system to re-establish balanced ocean chemical conditions and recover from human-induced acidification, and millions of years for coral reefs to return, based on records of natural
coral reef extinction events [7 & 8].
DEFINITION
This indicator illustrates the global mean average rate of ocean acidification, quantified by decreases in pH, which is a measure of acidity, defined as the hydrogen ion concentration. A decrease in pH value corresponds to an increase in acidity.
The observed decrease in ocean pH resulting from increasing concentrations of CO2 is an important indicator of change in the global ocean and the impacts of climate change.
This indicator provides information on:
- trends in ocean acidity measured at the Aloha station;
- yearly mean surface seawater pH levels reported on a global scale is computed from monthly pH values by
CMEMS.
ENVIRONMENTAL POLICY Acidification is addressed in the 2030 Agenda for Sustainable Development. One of the targets under Sustainable Development Goal (SDG) 14 (‘Conserve and sustainably use the oceans, seas and marine resources for sustainable development’), SDG 14.3, is to ‘Minimize and address the impacts of ocean acidification, including through enhanced scientific cooperation at all levels’.
On 4 March 2020, the European Commission proposed a European climate law to ensure a climate-neutral European Union by 2050 as a part of the
European Green
Deal. This law is designed to establish a basis for adaptable management, with focus on the implementation of mitigation measures, the monitoring of progress and the improvement of management approaches if needed.
REFERENCES
1. UN, 2015, 'Transforming our world: the 2030 Agenda for Sustainable Development', United Nations
(https://sustainabledevelopment.un.org/post2015/transformingourworld)
April 1, 2021.
2. Kahn, B., 2016, 'Earth’s CO₂ passes the 400 PPM threshold — maybe permanently', Scientific American 27 September
(https://www.scientificamerican.com/article/earth-s-co2-passes-the-400-ppm-threshold-maybe-permanently/)
April 19, 2021.
3. Rhein, M., Rintoul, S. R., Aoki, S., Campos, E., Chambers, D., Feely, R. A., Gulev, S., Johnson, G. C., Josey, S. A., Kostianoy, A., Mauritzen, C., Roemmich, D., Talley, L. D. and Wang, F., 2013, 'Observations: ocean', in: Stocker, T. F., Qin, D., Plattner, G.-K., et al. (eds), Climate change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, UK; New York, NY, pp. 255–316.
4. Joos, F., Frölicher, T. L., Steinacher, M. and Plattner, G.-K., 2011, 'Impact of climate change mitigation on ocean acidification projections', in: Ocean acidification, Oxford University Press, Oxford, pp. 272–288.
5. Helcom, 2013, Climate change in the Baltic Sea Area — Helcom thematic assessment in 2013, 137, Helsinki Commission — Baltic Marine Environment Protection Commission, Helsinki.
6. Bindoff, N. L., Cheung, W. W. L. and Kairo, J. G., 2019, 'Changing ocean, marine ecosystems, and dependent communities', in: Pörtner, H.-O., Roberts, D. C., Masson-Delmotte, V., et al. (eds), IPCC special report on the ocean and cryosphere in a changing climate, Cambridge University Press, Cambridge, UK.
7. Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R. M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R. G., Plattner, G.-K., Rodgers, K. B. et al., 2005, 'Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms', Nature 437(7059), pp. 681–686
(http://www.nature.com/nature/journal/v437/n7059/full/nature04095.html)
November 28, 2013.
8. Archer, D. and Brovkin, V., 2008, 'The millennial atmospheric lifetime of anthropogenic CO₂', Climatic Change 90(3), pp. 283–297 (http://www.springerlink.com/index/10.1007/s10584-008-9413-1) accessed June 27, 2012.
9. Carter, B. R., Williams, N. L. and Gray, A. R., 2016, 'Locally interpolated alkalinity regression for global alkalinity estimation', Limnology and Oceanography: Methods 14(4), pp. 268–277.
10. Carter, B. R., Feely, R. A. and Williams, N. L., 2018, 'Updated methods for global locally interpolated estimation of alkalinity, pH, and nitrate', Limnology and Oceanography: Methods 16(2), pp. 119–131.
11. Dore, J. E., Lukas, R., Sadler, D. W., Church, M. J. and Karl, D. M., 2009, 'Physical and biogeochemical modulation of ocean acidification in the central North Pacific', Proceedings of the National Academy of Sciences of the United States of America 106, pp. 12235–12240.
12. Dore, J. E., 2012, 'Hawaii Ocean Time-series surface CO₂ system data product, 1988-2008.', (http://hahana.soest.hawaii.edu/hot/products/products.html).
13. Copernicus Marine Service, 2021, 'Global mean sea water pH', Copernicus Marine Service
(https://marine.copernicus.eu/access-data/ocean-monitoring-indicators/global-mean-sea-water-ph)
April 1, 2021.
14. van Heuven, S., Pierrot, D. and Wallace, D., 2011, CO2SYS v 1.1: MATLAB program developed for CO2 system calculations. ORNL/CDIAC-105b, Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN.
15. UN, 2021, 'The Sustainable Development Agenda', Sustainable Development Goals, United Nations
(https://www.un.org/sustainabledevelopment/development-agenda/) April 1, 2021.
16. EC, 2020, Proposal for a regulation of the European Parliament and of the Council establishing the framework for achieving climate neutrality and amending Regulation (EU) 2018/1999 (European Climate Law), COM(2020) 80 final
17. EC, 2019, Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions ‘The European Green Deal’, COM(2019) 640 final of 11 December 2019.

THE
RIVER
NILE - Was then and is now the source of life for Egypt.
|